Microsize is a US-based pharmaceutical CDMO with over 30 years of specialized experience in micronization and particle size reduction. We help drug developers improve dissolution and bioavailability for poorly soluble and highly potent APIs, combining specialized containment, analytical support, and three FDA-inspected facilities to scale from development through commercial supply.
APIs Processed
Production Campaigns
Largest US Micronization Footprint
Years of Experience
Years of Successful FDA Inspections
Countless – Satisfied Customers
Contract Services
Particle Size Reduction:
Micronization & milling
Particle Classification:
Multiple screening technologies
Analytical Characterization:
Laser diffraction and morphology techniques
The Microsize Advantage
Decades of Experience
Hundreds of APIs processed in thousands of campaigns
Unparalleled Depth of Expertise
Pioneers in micronization technology
Largest US-based Footprint
Three facilities, >100,000 sqft, FDA inspected
World Class Containment Technologies
Hard wall and single use technologies for HPAPI
High Touch, Low Bureaucracy
Focused on relationship and speed
Enhanced Bioavailability: Accelerated
First-Choice Technology
Fastest approach to improving bioavailability and solubility
Streamlined Regulatory Pathway
Low regulatory risk; Implemented in numerous IND and NDA filings
Readily Scalable
Proven from grams to tons; limited variables of scale enhances speed
High Yielding
High throughput with minimal losses means competitive costing
Superb Precision/ Reproducibility
Narrow Gaussian PSD curves enhances quality and performance
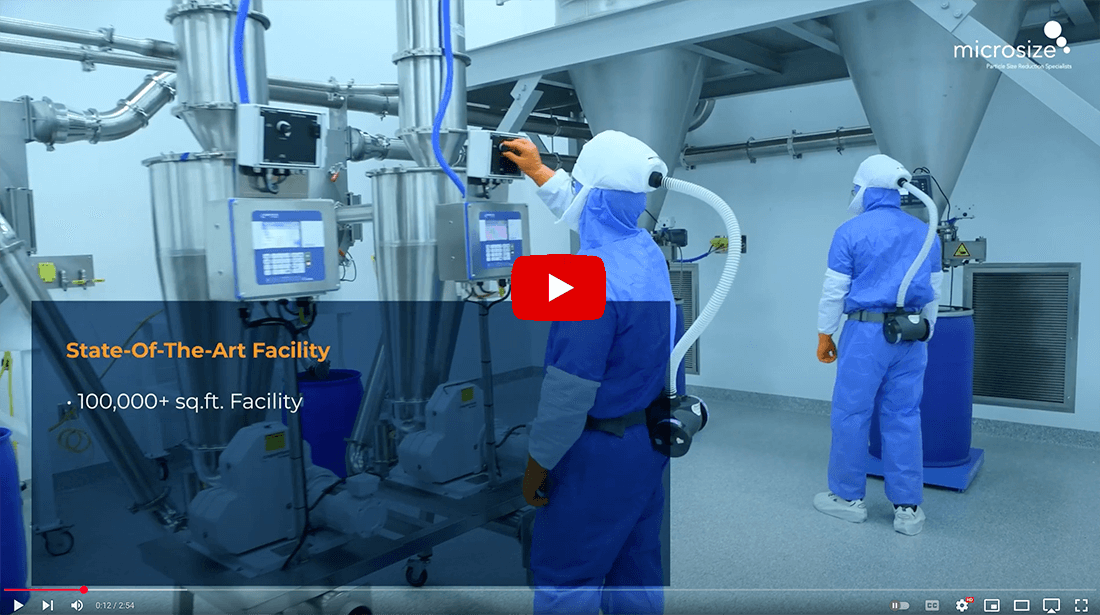
Operating from a 100,000 square foot, US-based, state-of-the-art, FDA inspected facility, Microsize has the experience and capabilities to rapidly develop, scale up, and process APIs and excipients ranging from grams to multi-metric tons, including highly potent compounds.
Microsize is THE partner of choice from biotechs to big pharma, and is recognized for its speed, responsiveness, and high customer-touch business model. When it comes to your toughest dissolution and bioavailability challenges...
FAQs
Q: What services does Microsize provide?
A: Microsize provides micronization and particle size reduction services to overcome dissolution and bioavailability challenges for small-molecule APIs and excipients, from feasibility through commercial production.
Q: What types of compounds can Microsize process?
A: Microsize handles hundreds of APIs and excipients, including highly potent and complex molecules, backed by thousands of successful micronization and particle processing campaigns.
Q: Why choose Microsize as a CDMO partner?
A: With over 30 years of experience, specialized containment, and three FDA-inspected facilities, Microsize delivers scalable, reliable micronization solutions that improve dissolution, bioavailability, and time to clinic.
Q: How does Microsize ensure quality and compliance?
A: Our three FDA-inspected facilities follow strict GMP standards, using advanced containment and analytical testing to consistently deliver safe, high-quality results.
