Analytical
Microsize has the analytical capabilities required to support early development and cGMP requirements. Our analytical experts work collaboratively with our clients during the analytical development stage to ensure complete alignment of expectations around analytical method development, validation, and release testing.
cGMP QA/QC
cGMP validated instruments, SOPs, and test methods to receive and release materials for clinical trials and commercial products
- FTIR Chemical Identification
- HPLC Cleaning Validation
- Loss on Drying (LoD)
- Karl Fischer Titration (KF)
- Particle Size Analysis (Malvern & Microtrac)
- Bulk & Tapped Density
Imaging and Particle Size Distribution
Supporting analytical method and process development
- Scanning Electron Microscope (SEM) Image Analysis
- Polarized Light Particle Size Imaging & Analysis (Nikon)
- Particle Size Analysis (Malvern & Microtrac)
cGMP QA/QC
cGMP validated instruments, SOPs, and test methods to receive and release materials for clinical trials and commercial products
- FTIR Chemical Identification
- HPLC Cleaning Validation
- Loss on Drying (LoD)
- Karl Fischer Titration (KF)
- Particle Size Analysis (Malvern & Microtrac)
- Bulk & Tapped Density
Imaging and Particle Size Distribution
Supporting analytical method and process development
- Scanning Electron Microscope (SEM) Image Analysis
- Polarized Light Particle Size Imaging & Analysis (Nikon)
- Particle Size Analysis (Malvern & Microtrac)
Solid-State Characterization
Benchmarking raw material and detecting post-milling changes
- X-ray Powder Diffraction (XRPD)
- Differential Scanning Calorimetry (DSC)
- Thermogravimetric Analysis (TGA)
- Dynamic Vapor Sorption (DVS)
Powder Flow Assessment
Anticipating and mitigating processing challenges before and after micronization
- FT4 Powder Rheometer
- Bulk & Tapped Density
- Angle of Repose
Solid-State Characterization
Benchmarking raw material and detecting post-milling changes
- X-ray Powder Diffraction (XRPD)
- Differential Scanning Calorimetry (DSC)
- Thermogravimetric Analysis (TGA)
- Dynamic Vapor Sorption (DVS)
Powder Flow Assessment
Anticipating and mitigating processing challenges before and after micronization
- FT4 Powder Rheometer
- Bulk & Tapped Density
- Angle of Repose
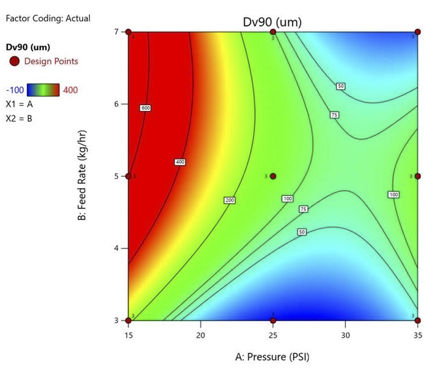
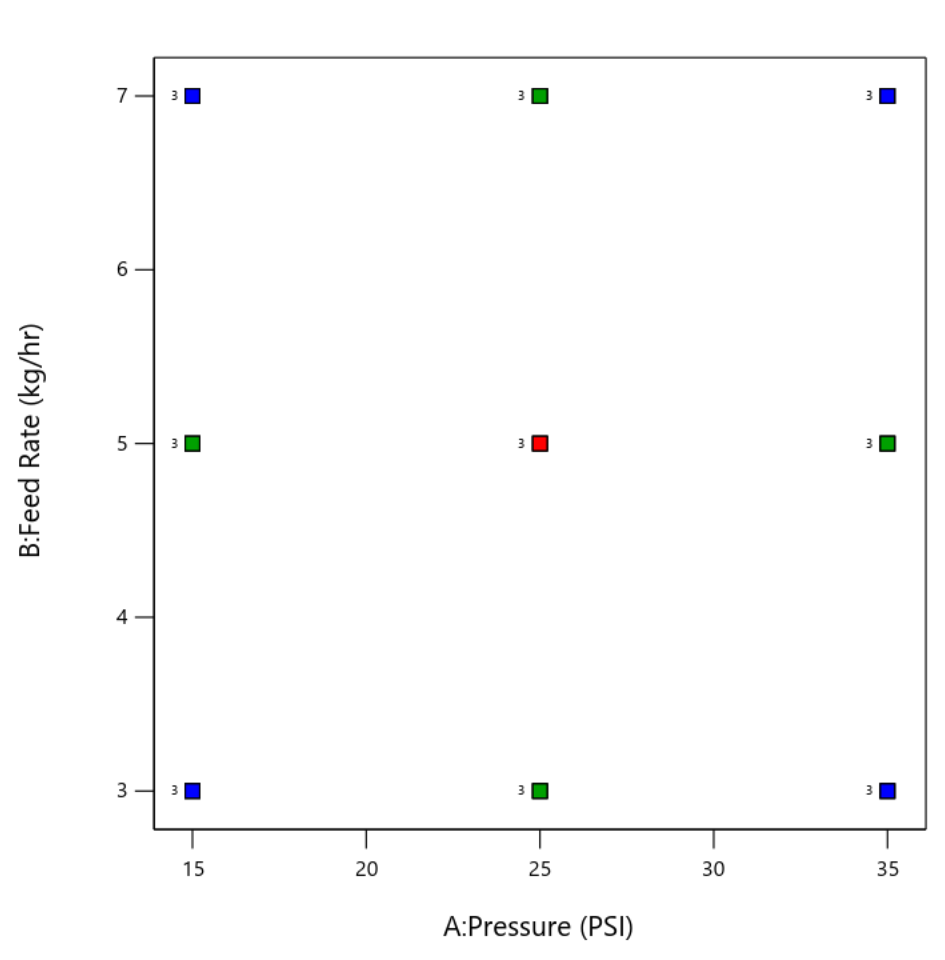
Design of Experiment (DoE)
Using analytics to gain deep understanding of the micronization processing parameters (CPPs) to ensure a robust, scalable, transferrable process to cGMP that meets defined critical quality attributes (CQAs)
- Utilizing Design Expert™ software by Stat-Ease to develop and optimize micronization processes
- Pairing statistics with science to drive decisions on process parameters (CPPs)
- Building central composite response surface models to predict particle size distribution (PSD)
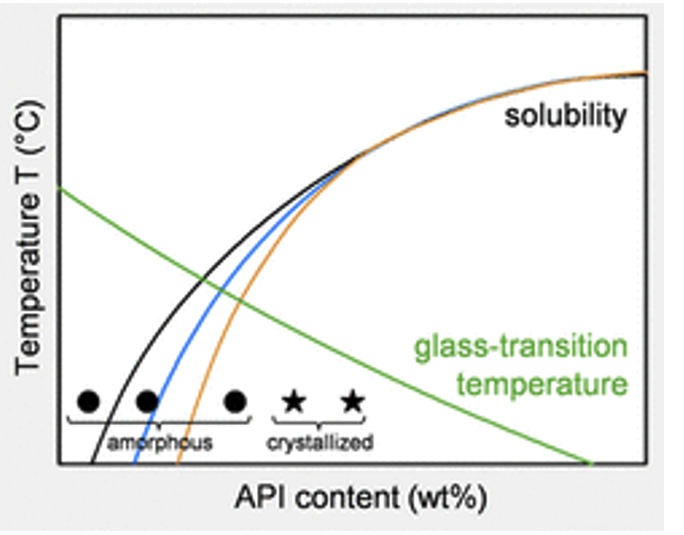
Short Term Stability Analysis
Evaluate physical stability of pre- and post-micronized material to assess potential for phase changes
- 1 – 4 weeks
- Ambient and elevated conditions
- Solid-state characterization and PSD determination
Design of Experiment (DoE)
Using analytics to gain deep understanding of the micronization processing parameters (CPPs) to ensure a robust, scalable, transferrable process to cGMP that meets defined critical quality attributes (CQAs)
- Utilizing Design Expert™ software by Stat-Ease to develop and optimize micronization processes
- Pairing statistics with science to drive decisions on process parameters (CPPs)
- Building central composite response surface models to predict particle size distribution (PSD)
Short Term Stability Analysis
Evaluate physical stability of pre- and post-micronized material to assess potential for phase changes
- 1 – 4 weeks
- Ambient and elevated conditions
- Solid-state characterization and PSD determination
Want to learn more about Microsize’s analytical capabilities? Want to discuss your analytical requirements? Contact us today.